Description
Best Quality Vacuum Reliable Blood Collection Needles
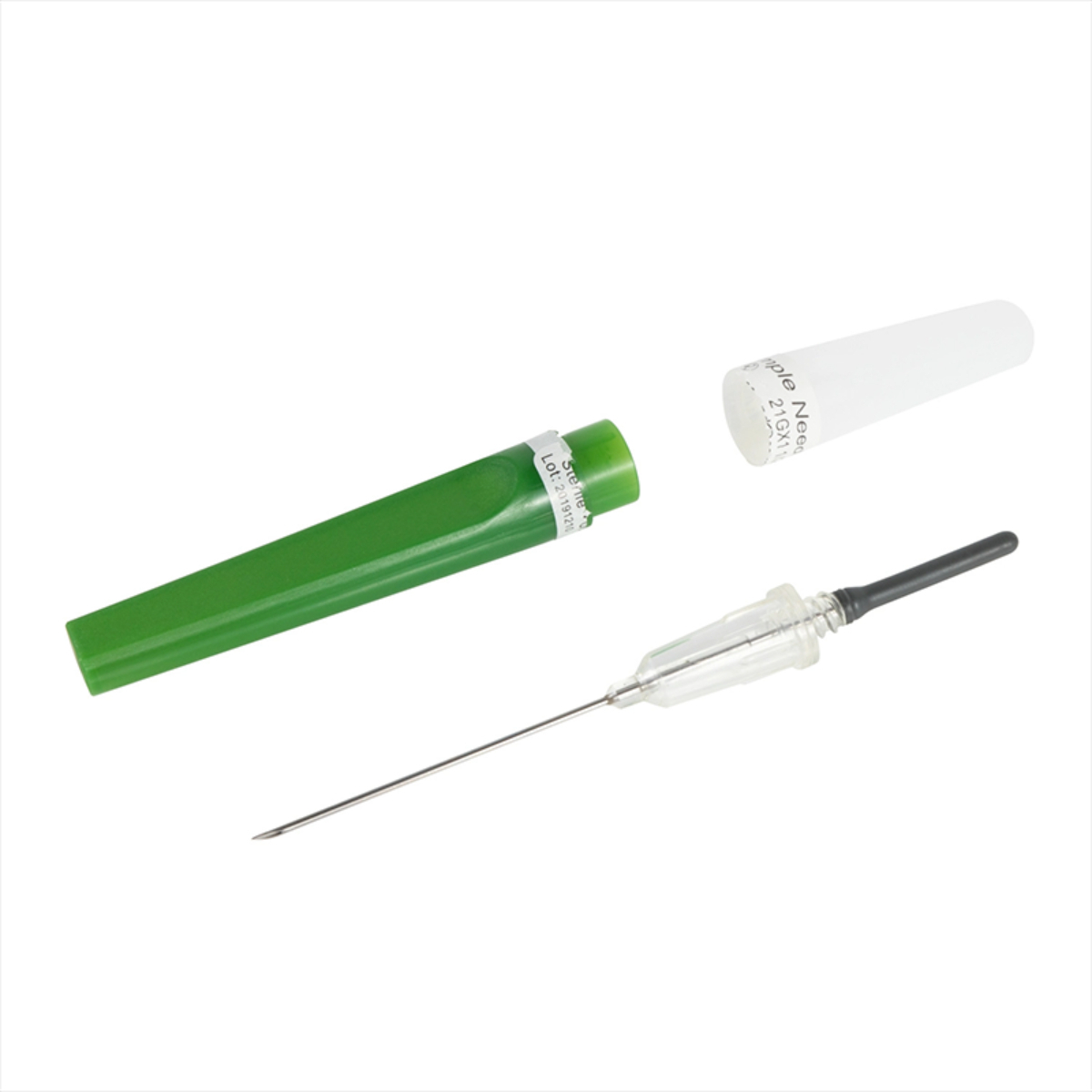
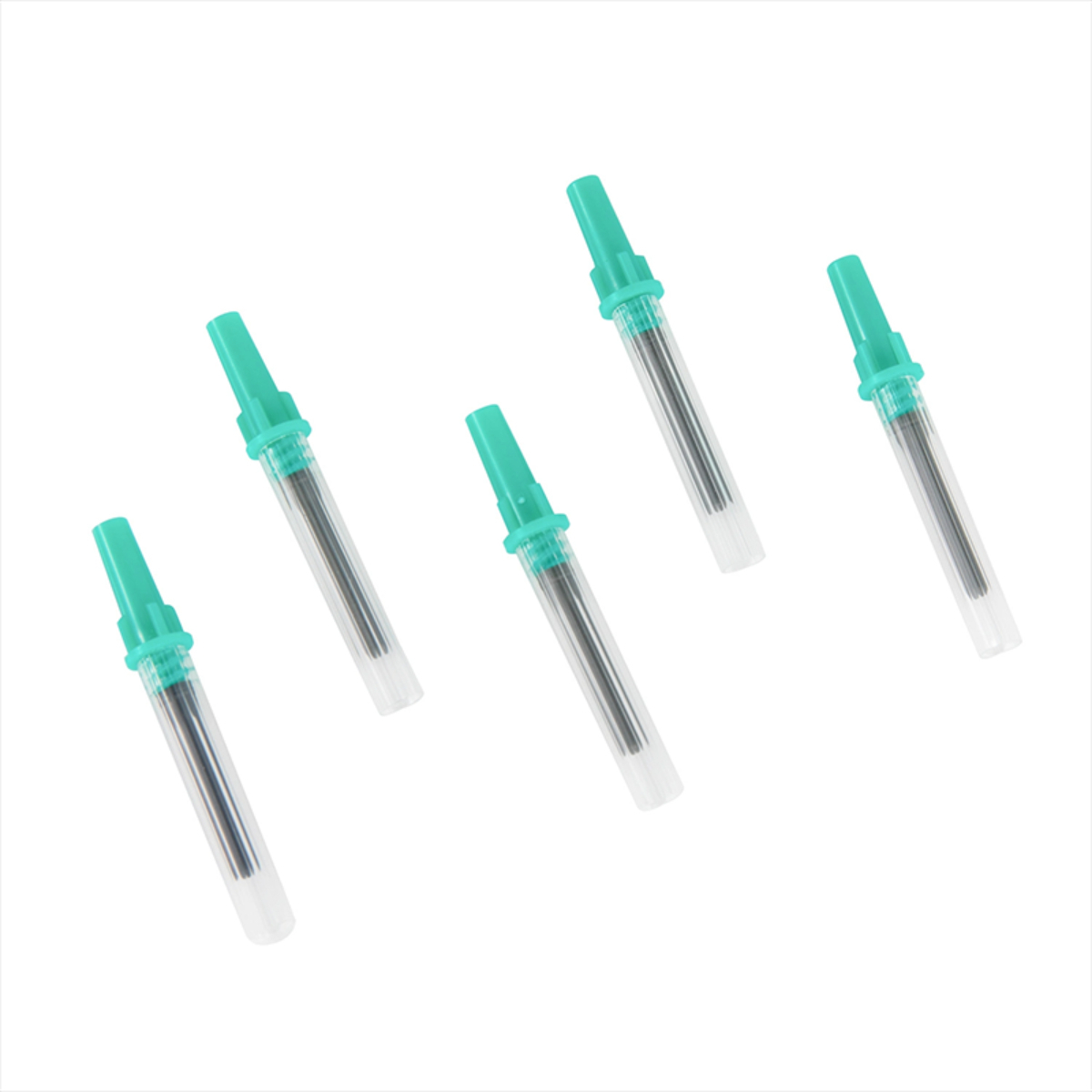
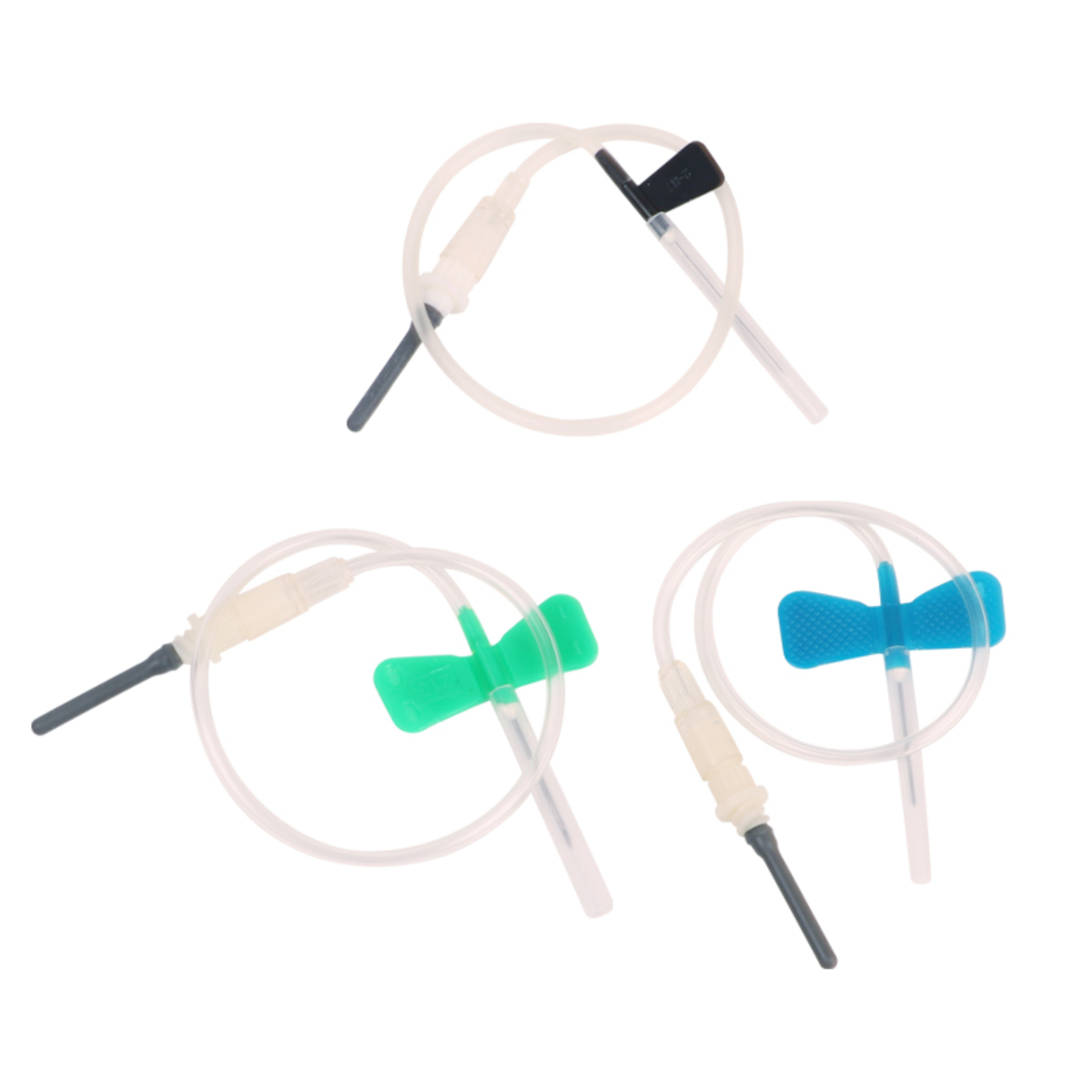
The Best Quality Vacuum Reliable Blood Collection Needles are precision-engineered medical devices designed to ensure safe, accurate, and hygienic blood sampling. Manufactured using high-grade medical stainless steel and advanced sterilization processes, these needles provide smooth vein penetration with minimal patient discomfort. Designed for compatibility with vacuum blood collection systems, they enable efficient, contamination-free blood draws in clinical and laboratory environments. Individually sterile-packed and easy to handle, these needles are ideal for hospitals, diagnostic laboratories, and healthcare professionals requiring consistent and reliable performance.
How to Use?
Using Best Quality Vacuum Reliable Blood Collection Needles is simple and safe. First, confirm that the needle packaging is intact and sterile before opening. Attach the vacuum blood collection needle securely to the holder. Insert the needle gently into the patient’s vein following standard phlebotomy procedures. Once the vein is accessed, insert the vacuum tube into the holder to initiate blood flow automatically. After collection, safely remove the needle and dispose of it in an approved sharps container to maintain strict infection control standards.
Key Features
• Designed for vacuum blood collection systems
• Ultra-sharp needle tip for smooth and painless vein penetration
• Manufactured from high-quality medical-grade stainless steel
• Sterile and single-use to prevent cross-contamination
• Ensures fast, efficient, and accurate blood collection
• Ergonomic design for better control and handling
• Individually packed in sterile blister packaging
• Compatible with standard blood collection holders
• Suitable for professional medical and laboratory use
Technical Specifications
| Specification | Details |
|---|---|
| Product Name | Best Quality Vacuum Reliable Blood Collection Needles |
| Type | Vacuum Blood Collection Needle |
| Usage | Single-Use |
| Needle Material | Medical-Grade Stainless Steel |
| Sterilization | Ethylene Oxide (EO) Gas Sterilized |
| Compatibility | Standard Vacuum Blood Collection Systems |
| Safety Feature | Single-Use Design Prevents Reuse |
| Packaging | Individually Sterile Blister Packed |
| Applications | Blood Collection, Diagnostics, Laboratories |
Applications & Use Cases
• Hospitals & Clinics – Routine blood sampling and diagnostic testing
• Diagnostic Laboratories – Contamination-free specimen collection
• Blood Banks – Donor blood collection procedures
• Emergency & Critical Care – Fast and precise blood draws
• Health Screening Programs – Mass testing and checkups
• Veterinary Clinics – Controlled blood collection in animals
• Research & Medical Studies – Clinical and pharmaceutical research
Advantages
• High reliability for consistent blood flow
• Reduced patient discomfort due to sharp needle design
• Maintains sample integrity with vacuum-based collection
• Minimizes infection risk with single-use sterile packaging
• Easy handling for healthcare professionals
• Compatible with standard vacuum blood collection systems
• Cost-effective for bulk medical procurement
Quality Assurance
• Manufactured under ISO and CE medical standards
• EO gas sterilization ensures complete hygiene
• Medical-grade materials ensure safety and durability
• Needle sharpness and strength tested for performance
• Batch-wise quality inspection for consistency
• Secure sterile packaging to prevent contamination
• Trusted by hospitals, labs, and healthcare institutions
Packaging & Logistics
• Individually sealed sterile blister packs
• Export-quality bulk cartons for safe transportation
• Clear labeling with batch number and expiry date
• Optimized carton size for storage and shipment
• Flexible MOQ options
• Worldwide shipping via air, sea, and land freight
• OEM/ODM branding and custom labeling available
Target Market
• Hospitals & Clinics
• Diagnostic & Pathology Laboratories
• Blood Banks & Collection Centers
• Government Health Departments
• NGOs & Medical Relief Organizations
• Medical Distributors & Wholesalers
• Veterinary Hospitals
• Research & Academic Institutions
Contact Us for Pricing
As a trusted manufacturer and supplier, we offer competitive factory-direct pricing and flexible bulk supply solutions. Contact our sales team today for quotations, samples, and fast dispatch support.